Part:BBa_K4140016
Cas12g
Part Description
RNA-guided Among its primary targets are single-stranded RNA substrates, Cas12g is a ribonuclease. Comparing it to other Cas12 proteins that have been found so far, CRISPS-Cas12g selectively detects RNA substrates, making it a potentially useful platform for transcriptome editing and diagnostics. While guided RNAs fold into a "F" shape that is primarily identified by the Rec lobes, a bilobed structure of Cas12g displays a tiny NUC2 and REC2 domain. To change the conformation of the REC and NUC lobes and activate Cas12g, target RNA and crRNA guide combine to form a duplex that is inserted into the cavity in the middle of the structure.
Usage
Cas12g is an RNA-guided protein and differs from other Cas12 proteins by targeting a single strand RNA substrates
Making it a potent platform for post-transcriptional modification so we use to control PAH and beta-galactosidase expression as it cleaves the mRNA of PAH and beta-galactosidase at a specific site without the need to recognize the PAM Sequence distinguishing it from other Cas proteins preventing the translation of PAH and beta-galactosidase just in case of overexpression of them or high level of tyrosine and absence of L7Ae.
Improvements
We improved this part BBa_K3743014 by:
Improvement by adding kink-turns
This year we employed this approach in our circuit as a regulatory domain, as we took advantage of Cas12g properties to control the expression of PAH in our therapeutic circuit as it differs from other Cas12 proteins by targeting a single-strand RNA substrate making it a potent platform for post-transcriptional modification, and reduces the chance of gene knockdown as it inhibits translation not the transcription by nucleotide deletion like other Cas protein due to inactivation of its NUC domain which mediates the nuclease activity of Cas protein. We carried out an improvement to its functionality limiting its off-targeting effect by adding a kink turn upstream to the 5’ end of its mRNA (translation initiation site) as shown in figure 1, which acts as a translation switch for Cas12g by repressing its synthesis in the presence of L7Ae due to the formation of a crystal structure composed of L7Ae-boxC/D k-turn complex which inhibits ribosomal function on the mRNA of Cas12g but in the absence of L7Ae protein Cas12g is freely expressed. The presence or lack of L7Ae is correlated with the concentration of phenylalanine since TyrR dimer, ATP, and the paroF promoter are required for L7Ae's production.
Improvement by directed evolution
After performing mutagenesis prediction of mutational landscape of cas12g and tested the effect of these mutations on the evolutionary fitness of the protein after generating multiple sequence alignment of the protein sequence and predict mutational landscapes. As shown in the chart, the (R627W) mutation showed the highest score compared to other mutations. On the contrary, we can see that the (L654F) contributed to the lowest evolutionary fitness to cas12g.As shown in Figure (5)
Characterization by mathematical modeling
This model is to simulate the kinetics of the riboswitch (L7Ae with kink turns) that is used in our circuit. The designed circuit is to detect if the increasing substance is either phenylalanine or tyrosine via TyrR. So if phenylalanine level is elevated, L7Ae is formed as it is downstream TyrR that forms a complex via binding with its kink-turn on another circuit; that complex inhibits expression of cas12g, so the circuit will be able to express lacZ alpha (beta-galactosidase) in diagnostic circuit or PAH in the therapeutic circuit. If tyrosine level is elevated with a decreased level of phenylalanine, it activates tyrR inhibitory promoter so no L7Ae would be expressed resulting in cas12g expression to control the circuit as shown figure (4) and graph (1).

Figure (4) illustrates the kinetics of all reactions in riboswitch model

Graph (1) illustrates riboswitch kinetics in which Q represents the condition where L7Ae is expressed and bound to its kink-turns ,therefore inhibiting the expression of cas12g. However, M represents no expression of L7Ae in which cas12g would be expressed to control the circuit if the phenylalanine is absent.
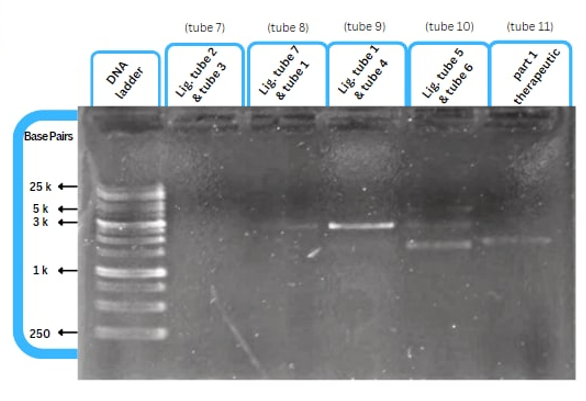
Experimental Characterization
This figure shows an experimental characterization of this part as it's validated through gel electrophoresis as it is in lane 4. The running part (ordered from IDT) included Human u6 Promoter - -Kinkturn - CMV Promoter - Cas12g.
References
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 313
Illegal PstI site found at 787
Illegal PstI site found at 1729 - 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 313
Illegal PstI site found at 787
Illegal PstI site found at 1729 - 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 1308
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 313
Illegal PstI site found at 787
Illegal PstI site found at 1729 - 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 313
Illegal PstI site found at 787
Illegal PstI site found at 1729
Illegal NgoMIV site found at 1230 - 1000COMPATIBLE WITH RFC[1000]
| None |